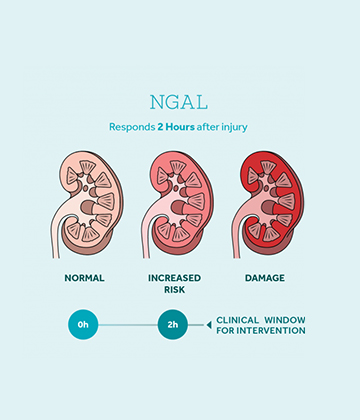
Serum Creatinine Vs NGAL
October 1, 2022For many decades, one of the most accepted and conventional point-of-care diagnostic platforms has been Lateral Flow immuno-assay (LFIA). During COVID pandemic, LFIA based rapid antigen tests were the most used screening test platforms at point-of-care. For many years, the development of LFIA tests for use in developing countries has focused mainly on infectious diseases, including HIV. World Health Organization (WHO)/UNAIDS estimates that more than 5 million people received antiretroviral therapy, and more than 29 million people were tested for HIV infection in developing countries in 2010, due to use of the LFIA platform. Currently, many of the diseases covered in “Second WHO Model List of Essential In Vitro Diagnostics”, published in 2019 and “The selection and use of essential in vitro diagnostics” published in 2021 does not have product recommendation in the section of “WHO prequalified or recommended products” specially cancer biomarkers like Alpha-Fetoprotein, Beta HCG, Prostate specific Antigen (PSA) etc. and few inflammatory markers like calprotectin, C-Reactive Protein. This is essentially because of the absence of a platform or technology that has high penetration. LFIA has high penetration and is a scalable platform that is ease-to-use. Although LFIA is a very scalable platform, however, it is qualitative in nature. LFIA has been made quantitative by combining its use with a reader or an analyser. Such tests require the healthcare facility to have a specific laboratory infrastructure and skilled manpower to perform these tests making these tests expensive and out of reach for people to get themselves treated at government hospitals, in smaller towns or villages.
On the other hand, digital technologies like the internet, mobile phones, and all the other tools that can collect, store, analyse, and share information digitally have spread quickly. More households in developing countries own a smartphone or a mobile phone than have access to electricity or clean water, and nearly 70% of the population at the bottom of economy pyramid in developing countries own a smartphone. The number of internet users has more than tripled in a decade, from 1 billion in 2005 to around 3.2 billion at the end of 2015. Smartphones and the internet have deep penetration even at the bottom of the economy pyramid and the same is true for conventional Lateral flow immuno-assay in the healthcare system.
QAWaCh Bio’s innovative platform called ‘Q-PLAT’ uses a combination of ultra-sensitive lateral flow immuno-assay (LFIA) and smartphone application to quantify LFIA results and making diagnostic testing easy, personalized, and cost-effective. This innovation will bring together the power of smartphone and compact testing platform like LFIA and giving point-of-care solution for biomarkers such as cancer biomarkers even in the primary healthcare setting. In case, of no electricity and absence of trained manpower this innovation can work with minimum infrastructural requirements. QAWaCh Bio’s innovation not only provides accurate measurement of the critical analyte but can also work in semi-quantitative way (in the absence of smartphone) in conditions like floods, natural calamity and war, thus, making it a zero-power innovation. Q-Plat can be used by primary healthcare workers or ASHA workers, requiring no specific laboratory skills for the handling of these tests. Currently, there are many diagnostic LFIA readers available in market specially from developed countries however, all of them require an expensive analyser with infrastructural requirements. The impact of our innovation will be directly measured by looking at the number of downloads of mobile application in future in the developing countries and the sales made in these areas.